Unlocking the true potential of Image Guided Ablation Therapy
Designed for Real time Predictive visualization of ablation tissue response
Ablation Tissue Response Visualized.
The innovative BioTrace Technology designed to predict and visualize the 24-hour post-treatment ablation tissue response in real-time
Allowing physicians to see where they have previously been blind
BioTrace™ is designed to interface with commercial ultrasound imaging systems. Its core innovation is in tracking the tissue’s unique “biological signature” when responding to heat during thermal ablation. This signature will be used to predict and visualize the post 24-hour thermal effect with the goal of optimizing the surgeon’s level of control and accuracy during the procedure, to minimize healthy tissue damage and maximize target tissue ablation.
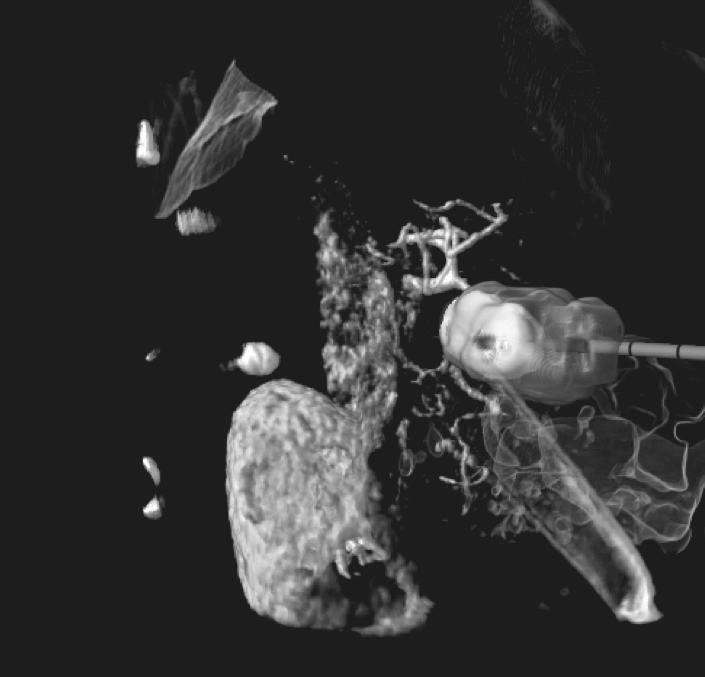
A comprehensive all-around system
An A to Z Solution, the BioTrace™ technology is designed to affect and improve all stages of patient care, from PSM, through planning & simulation to real-time lesion imaging & efficacy analysis
The information on this website regarding the BioTrace technology is for demonstration purposes only. The BioTrace technology as presented in the website is limited by U.S. law to investigational use.
Providing vision. Aimed at Optimizing results.
A must have system for optimal ablation
Efficacy
Designed to maximize efficacy and technical success
Safety
Designed to avoid overtreatment and increase safety
Cost
Designed to be cost effective. No hardware necessary
Ease of Use
Designed to seamlessly integrate to existing workflow
Clinical Study Case Examples
One real-time image is better than a thousand simulations
The provided cases performed in collaboration with Dr. Ryosuke Tateishi, University Tokyo Hospital
-
CASE 01
- 61 Year-Old Patient
- RFA Ablation
- Tumor Size 1.8 cm
- Liver Segment #3
- Ablation time: 3 min
92%
BioTrace™ accuracy
compared with 24h post CECT
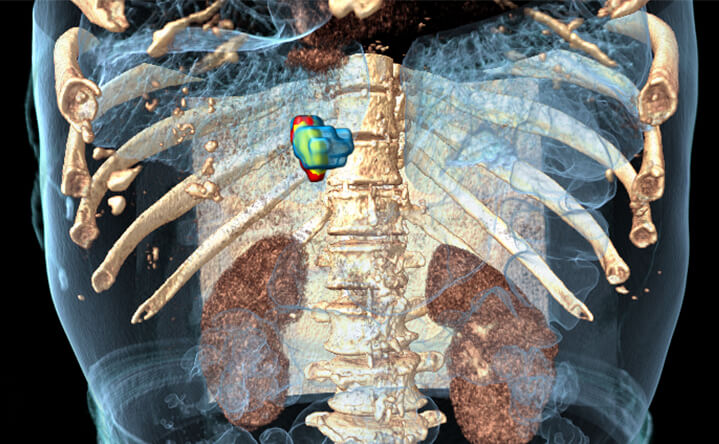
92%
BioTrace™ accuracy
compared with 24h post CECT
-
CASE 02
- 58 Year-old Patient
- RFA Ablation
- Tumor Size 2.2 cm
- Liver Segment #6
- Ablation time: 2.4min
91% BioTrace™ accuracy
compared with 24h post CECT
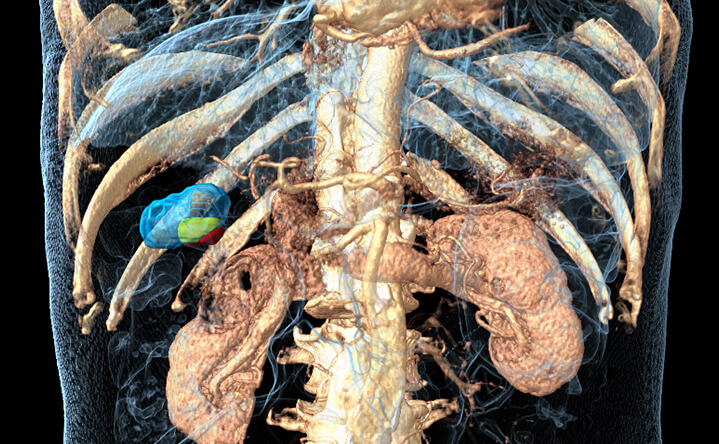
91% BioTrace™ accuracy
compared with 24h post CECT
-
CASE 03
- 63 Year-old Patient
- MW Ablation
- Tumor Size 1.5 cm
- Liver segment #4
- Ablation time: 2min
90% BioTrace™ accuracy
compared with 24h post CECT
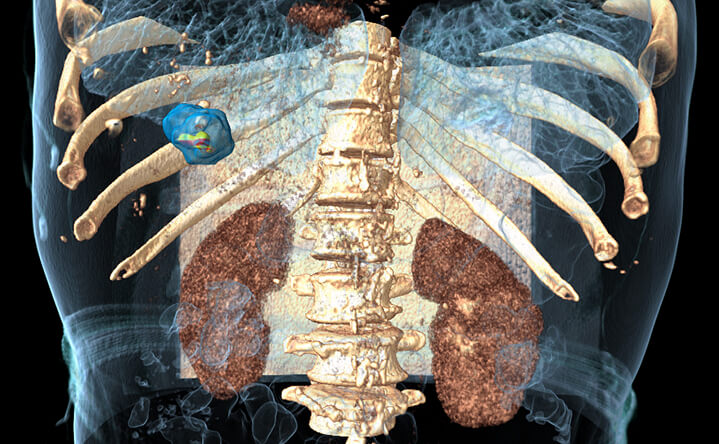
90% BioTrace™ accuracy
compared with 24h post CECT
Transforming ablation treatments into guided precision therapies
Current Application
Oncology Liver tumor ablation
Advancing thermal ablation into the first line treatment option for tumor removal
Pipeline
Cardiovascular
Transitioning from complicated guess-work into precise, real-time feedback dependent treatments
Pain management
Dramatically increase safety in high-risk nerve-ablation procedures
Team











Board of Directors
Board Director
MR. YOSSI ABU
CEO, NewMed Energy
Director
MR. YOSSI ABU
Founder & CEO, TechsoMed
Director
MR. EIJI KAKIUICHI
Chairman, SCREEN Holdings
Director
MR. TODD KALOUDIS
Managing Director, Cobro Ventures
Director
MR. FRED SHANE
Managing Partner, Axil Capital
Director
MR. WANG CHONG
Executive Director, Yonghua Capital
Partnerships
Investors







Partners







Clinical







News

December 2022
TechsoMed announces the opening of a new subsidiary in Bremen with Fraunhofer Mevis to accelerate End-to-End solutions for Image-Guided Thermal Ablation therapy

October 2022
TechsoMed announces completion of full enrollment in pivotal US trial, evaluating its BioTraceIO software for liver ablation outcomes assessment in liver cancer patients
Contact info
TechsoMed
Tel: +972-8-6198866
Email: info@techsomed.com
Meir Weisgal 2, Rehovot, Israel
© 2023 Techsomed All Rights Reserved | Website by Pearlcom.co.il
Read our Privacy Policy
© 2021 Techsomed All Rights Reserved
Website by Pearlcom.co.il
The information on this website regarding the BioTrace technology is for demonstration purposes only.
The BioTrace technology as presented in the website is limited by U.S. law to investigational use.
MK000014-001





